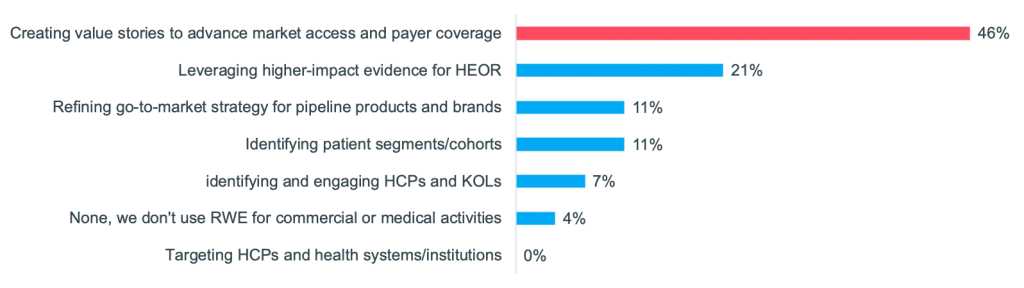
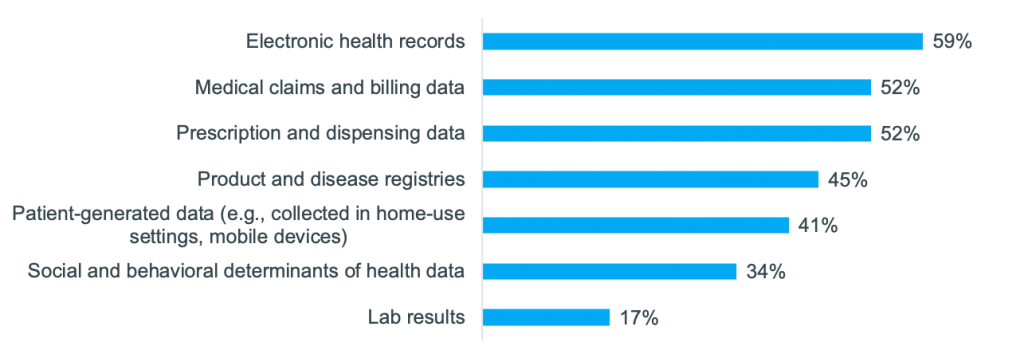
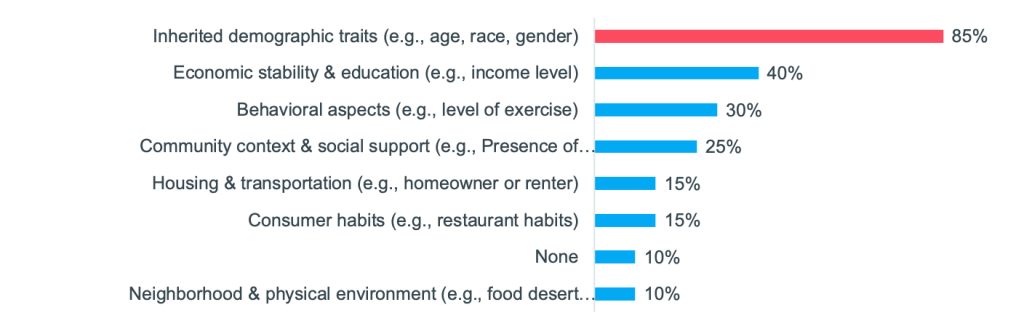
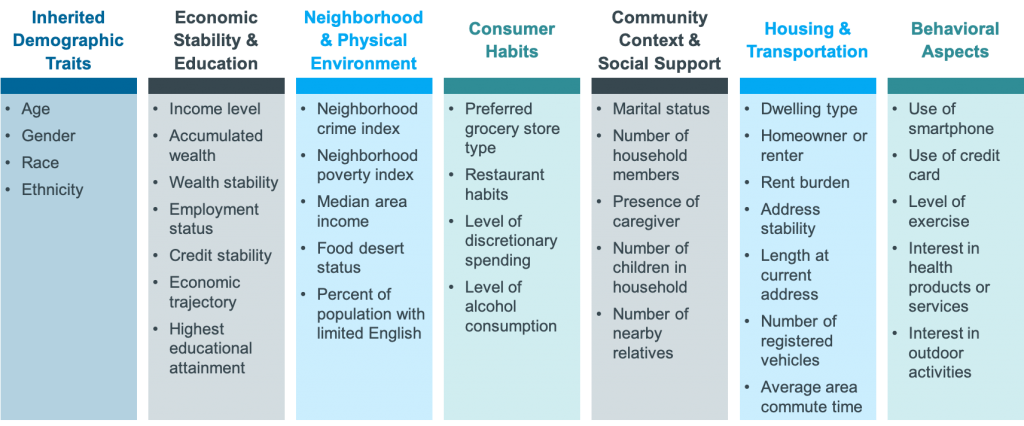
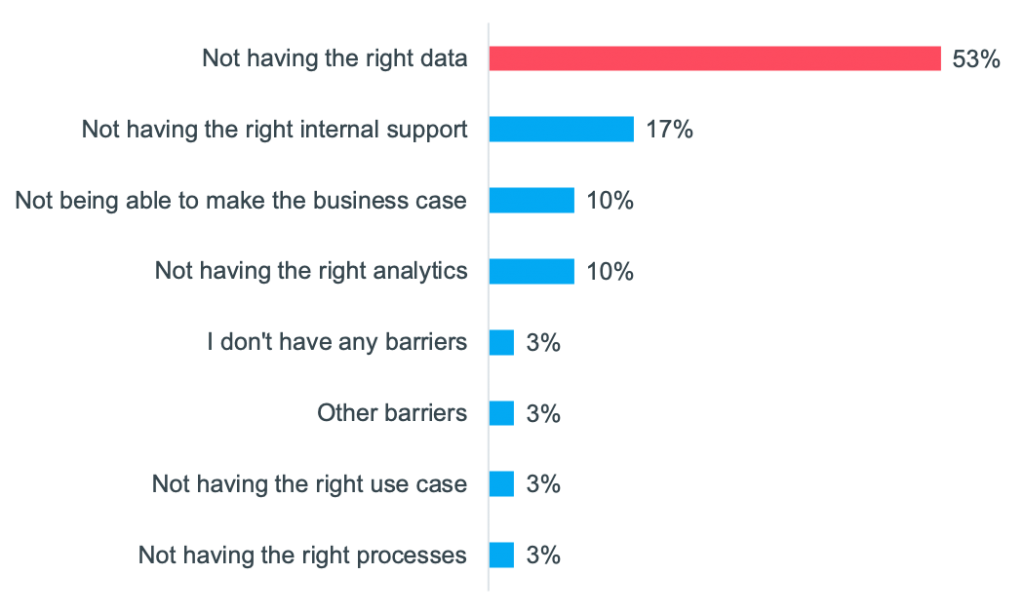
On Tuesday, May 25, 2021 we hosted a workshop on “Using RWE to drive next-level commercial impact.” It was attended by 35 commercial, HEOR, and RWE experts, from over 25 different top 50 pharma companies. Manisha Shetty Gulati, COO, Clarify Health and Paul Petraro, Global Head Real World Evidence (RWE), Analytic Center of Excellence, Boehringer Ingelheim, co-led the discussion. Participants shared how they are using RWE today and their goals for the future. “The key is that we start thinking about real-world evidence as early as possible, and it needs to be a constant,” said Paul as he set the stage for the session. He shared how Boehringer Ingelheim is currently collaborating with Clarify, leveraging the company’s RWE software to put the “start early” approach into practice. “With Clarify, we have access to their software, offering us a single source of truth for RWE that we can come back to time and again throughout the lifecycle.” Paul went on to explain that he’s focused on using RWE across the entire lifecycle of a molecule from development through line extensions. For an upcoming drug launch, Boehringer Ingelheim is using Clarify’s RWE software to strengthen its payer value messaging, better focus its HCP outreach, and refine its HCP targeting. Paul is putting his start-early approach into practice, ensuring that as soon as marketing authorization is reached, they’ll be prepared to hit the ground running. Paul compared how pharma has traditionally used RWE to the approach he’s institutionalizing at Boehringer Ingelheim. Typically, in Phases I and II, he said, “we would think about the epidemiology of the disease and the disease burden, put it aside, and then come back and start thinking about it again at launch. Traditionally we might not need as much evidence early on, but as we reach launch and immediately after launch the need increases. The way I want to start thinking about it is: early and constant.” In a Zoom poll, participants were asked, “for which of these commercial and medical activities do you rely on RWE the most today?” 46% said they rely on it the most for creating value stories to advance market access and payer coverage. Paul reacted: “That is definitely where we think about traditional real-world evidence—it’s focusing on payer coverage and the value proposition.” In another poll, the audience was asked, “what types of real-world data are most important to demonstrate value to payers and providers in addition to clinical trial data?” (They could select multiple choices). The top answer was electronic health records (EHRs); however, all these data were important to varying degrees. One participant shared why she selected EHRs. It is because she’s most interested in patient outcomes, comorbidities, and the impact of therapies on patients. Another participant shared her reasoning: it is easier to center payer discussions around clinical data. But she said that if the question had asked about using RWE to support discussions with patient organizations or patients themselves, then she would have selected more patient-centric data, such as social and behavioral determinants of health (SDoH). Participants were then asked, “What types of SDoH data do you use today?” 85% said they use inherited demographic traits, while other types of SDoH factors were less commonly used. However, all participants agreed that understanding a patient’s journey, inclusive of medical, social, and behavioral factors is a more precise way to identify the right patient for the right therapy at the right time. Sample of SDoH attributes that decorate Clarify’s patient journeys There are many factors beyond inherited demographics traits that impact a patient’s journey. Below are examples of the SDoH factors that decorate Clarify’s patient journeys. While participants were interested in using many of these data, a common challenge was not having access to the data and not being able to use it in a de-identified way to draw patient-level insights. Many of the pharma companies agreed that they have lots of data—from clinical trials and other sources—but RWE is not readily accessible. Manisha talked about how “oftentimes RWD are in disparate, siloed data sources,” which makes it hard to extract the evidence and insights needed. We asked the attendees, in another poll, “What is your biggest barrier to leveraging RWE today?” 53% said not having the right data is their biggest barrier and 17% said that not having the right internal support is. One participant explained that often the data needed to build her case is not actually captured. Even though millions of data are captured in registries, it is not always the right data to provide meaningful results. For example, she spoke about longitudinal studies and wanting to use RWD to adjust for medication, dose, or enzyme levels, but that data is not always available. Another participant said that her challenge to getting the right data centered around patient privacy and getting access to these data. While patient consent is used to collect clinical trial and other data, Manisha described another way, which is to de-identify the data. “At Clarify, we tokenize patient data from different sources so that they’re de-identified, and then we link them together at the patient level. Using our software applications, you’re able to see a 360-degree view of every patient journey, but the patients are de-identified.” Learn more about Clarify’s RWE software, Clarify Growth and Clarify Portfolio, which support commercialization across the product lifecycle. These cloud-based analytics software products put RWE to work faster. Users can instantly query over 300M SDoH-decorated patient journeys for precision insights, which can: uncover underdiagnosed patients, pinpoint HCPs who are treating patients in need of second-line therapy, and track competitor market share at the patient-cohort level. Here are some additional resources on real-world insights for life sciences: Light up the patient journey with self-service analytics Clarify Health Launches Real-World Evidence on Health Disparities
Today’s RWE use cases
Demonstrating value to payers and providers
Real-world insights into socio-behavioral determinants of health
Barriers to leveraging RWE
- Author Details


