
From FDA regulatory approvals to commercialization strategies to value demonstrations, real-world evidence (RWE) plays a pivotal role in the product lifecycle of diagnostics, medical devices, and digital therapeutics (DTx). The FDA published Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices to provide guidance on leveraging real-world data in regulatory decision-making within the medical device industry. Life science analytics software can deliver keen insights into RWE for optimized market access opportunities and better go-to-market plans and commercial strategies.
How RWE supports the product go-to-market strategy for medical devices
Building a robust commercialization strategy is critical for device products that seek to penetrate new or existing markets. Successful go-to-market (GTM) plans should be targeted and incorporate internal stakeholders’ perspectives that leverage RWE to provide insight into the market landscape, target audiences, claims and evidence, and sales and marketing:
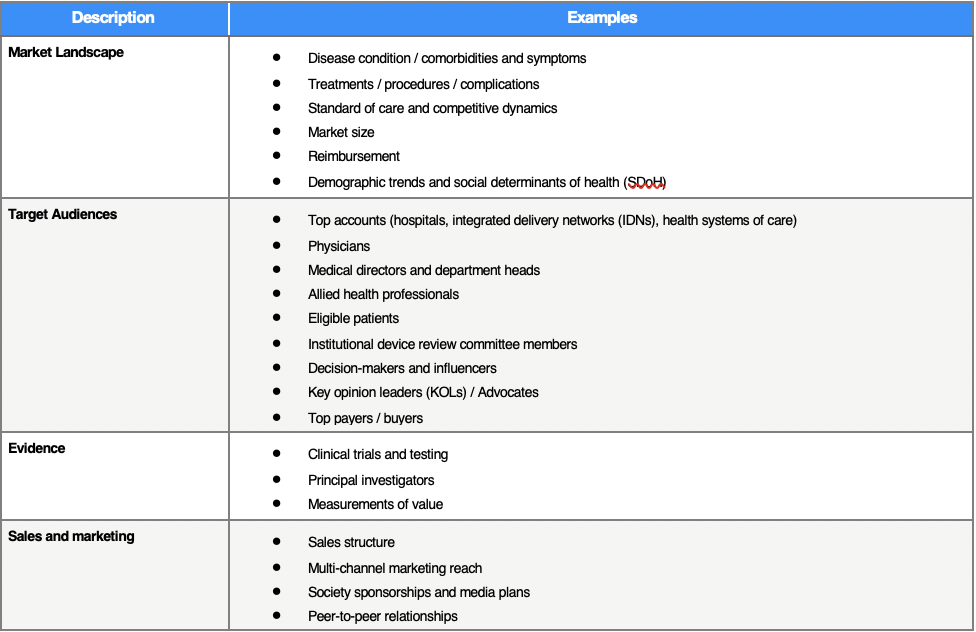
Additionally, for each device area in their portfolio, companies must define what value means to each of their stakeholders (i.e., health systems, IDNs, payers, HCPs, patients) and demonstrate that value to them. Robust clinical and economic RWE can support value-based pricing and innovative risk-sharing contracts for medical device manufacturers. To collect and report meaningful, measurable outcomes data, medical device companies may need to invest in a healthcare analytics infrastructure with the capability to deliver actionable insights.
Investing in the right technology stack to enable easy access to actionable insights
The Clarify Atlas Platform is the healthcare industry’s most precise and actionable source for patient journey insights. Built on a securely linked dataset covering over 15 billion government and commercial medical and Rx claim records, clinical data, and 400+ social determinants of health factors. Atlas maps over 300 million individual patient journeys to deliver 18+ billion AI-powered predictions that can be explored on-demand through self-service software. Clarify’s cloud-based analytics software provides access to precise insights for multiple stakeholders use cases, allowing them to:
- Test clinical and business development questions about patient outcomes
- Understand physician and account referral patterns to craft campaign messaging
- Support market access and strategic planning across the product lifecycle
- Develop internal account planning and performance methodologies
- Optimize external customer dialogues and communications through local markets volume insights
Clarify’s RWE software in action
Applications of Clarify’s solutions span departments – ranging from clinical teams seeking to identify clinical research sites and principal investigators for trials to commercial teams providing education to HCPs. Let’s take a closer look at three common use cases.
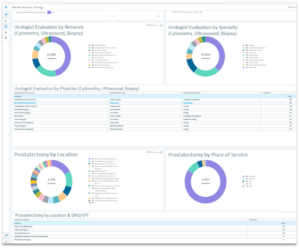
Test clinical and business development questions about patient outcomes
With Clarify’s software, you can continuously refine strategies and tactics by ideating concepts, validating them via real-world data, and following patient populations through the treatment continuum to assess outcomes.
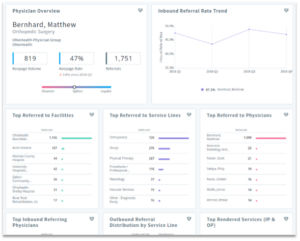
Understand physician and account referral patterns to craft campaign messaging
Leveraging analytics on referral patterns by account and HCP allows life sciences teams to devise marketing campaigns and educational messaging to the right influencers.
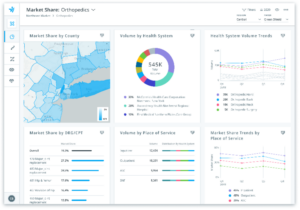
Optimize external customer dialogues and communications through local markets volume insights
By arming field teams with local market insights, they can better engage their customers. Clarify’s analytics offer insights into accounts and HCP volume and market share trends.
Clarify has supported genetic testing companies, digital therapeutics manufacturers, and device companies across varying therapeutic areas, including women’s health, oncology, and behavioral health. Click here for more information on use cases and to schedule your demo today.











